Group Kaaij
The Kaaij group studies how the organization of the 3D genome is regulated and how its architecture impacts the regulation of gene expression.

Research uitklapper, klik om te openen
Throughout the process in which a fertilized oocyte gives rise to a full-grown organism the spatiotemporal regulation of gene expression is of pivotal importance. Recent efforts from the ENCODE consortium led to the identification of more than two million loci in the human genome with enhancer characteristics. This means that there are approximately hundred times more enhancers than genes in the human genome and it is currently poorly understood how gene-enhancer specificity and function is achieved. Nevertheless, diverse gene expression patterns and fine-tuning of spatio-temporal gene expression in cells are thought to be the result of differences in the regulatory landscape a gene resides in. The importance of this fine-tuning is exemplified by studies that identify mutations that are linked to disease states, called genome wide association studies (GWAS). Collectively, these studies result in the realisation that many disease-linked mutations are actually located in the non-coding genome. More specifically, many of these mutations are found in distal regulatory sites such as enhancers and CTCF binding sites.
In the nucleus DNA is organized at different levels. Chromosomes typically occupy a particular part of the nucleus known as chromosome territories. Furthermore, DNA is segmented in genomic regions that preferentially self-interact called topological associated domains (TADs) (Figure 1a and 1b). Two key players in the formation of TADs have been identified: The transcription factor CTCF and the Cohesin complex. TAD boundaries are demarcated by CTCF and it is thought that the Cohesin complex extrudes DNA through its lumen and specifically halts at CTCF binding sites (Figure 1c). The functional importance of TADs and loops is not clear, but an attractive hypothesis is that TADs play a role in simultaneously facilitating and restraining the interaction between regulatory elements. Indeed, disruption of TAD boundaries leads to novel promoter-enhancers interactions and differential gene expression. However, despite the vast amount of genome wide genomics datasets, the field of 3D genome organization is still in its infancy and the basic concepts and molecular mechanisms are still poorly understood.
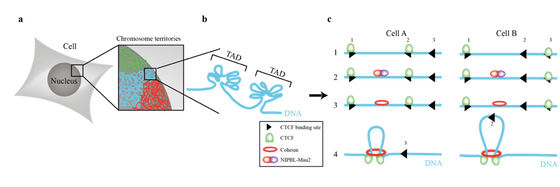
Schematic representation of how DNA is structured in the nucleus (a) Overview of how DNA is organized at the whole chromosome level (b) Genome organization at the level of TADs (100kb-mb) (c) Individual DNA loops generated by Cohesin and Ctcf. (1) The Ctcf binding site selection conundrum in different cell types. (2) Shows the NIBPL-Mau2 dimer that loads Cohesin on chromatin (3) The moment right after Cohesin has been loaded on chromatin (4) The loops that have been generated after Cohesin loop extrusion has finished and Cohesin is stalled by Ctcf bound to chromatin. Comparing cell A and B shows the influence differential Ctcf binding has on loop anchor position.
Research questions uitklapper, klik om te openen
- The fundamental questions that we are eagerly investigating in the lab are:How are cell type-specific DNA loops generated while CTCF and the Cohesin complex are both constitutively expressed?
- How dynamic are the processes related to 3D genome organization?
- How does ADNP contribute to the clinical manifestation of Helsmortel-van der Aa syndrome?
Experimental approach uitklapper, klik om te openen
In order to address these questions in the Kaaij group, we combine wet lab techniques with computational approaches. Most of our research is done in mouse embryonic stem cells, which are routinely manipulated using CRISPR-Cas9 to generate transgenic cells relevant for our research. Other routinely used techniques are (crosslinking-)IP mass-spectrometry to identify protein-protein interaction partners, 4C-seq and Hi-C to probe for chromatin interactions, ChIP-seq, ATAC-seq, NOMe-seq (Figure 2) and RNA-seq to monitor DNA binding of transcription factors and gene expression.
Our research will impact our molecular and developmental understanding of 3D genome architecture. Ultimately, more detailed insight into the mechanisms that shape the 3D genome will be essential to understand and eventually treat diseases with an epigenetic basis.
Areas of expertise uitklapper, klik om te openen
Throughout his scientific career Lucas Kaaij has been captivated by the observation that seemingly identical cells with the same genetic material can give rise to a multicellular organism containing numerous different cell types with highly specific functions. Epigenetic mechanisms play an important role during the differentiation of cells to specific cell types and Lucas Kaaij has been studying various aspects of this process in the course of his PhD and postdoctoral research.
During his PhD in the group of René Ketting at the Hubrecht Institute in the Netherlands, he quantified DNA methylation dynamics throughout differentiation of the mouse small intestinal stem cell in collaboration with the laboratory of Hans Clevers. This work revealed that differentiation in the small intestine is accompanied by very limited DNA methylation dynamics. Although surprising at first this finding helped to understand the remarkable plasticity observed in the small intestinal system.
After his PhD, Lucas did a short interdisciplinary postdoc co-supervised by Alexander van Oudenaarden (Hubrecht Institute) and René Ketting (IMB, Mainz), to study a zebrafish mutant with a partial penetrant germ cell phenotype. To approach this as unbiased and as comprehensive as possible, he used single-cell RNA sequencing (scRNA-seq). By generating and analyzing the scRNA-seq data from wild-type mutant germ cells he discovered that the mutant does not balance the mRNA levels of key germ cell specification factors correctly.
During his second postdoc, in the group of Marc Bühler at the FMI in Basel, Lucas studied the mechanisms and the functions of 3D genome organization. The genome is segregated in topologically associated domains (TADs), which are regions in the genome that preferentially self-interact. The transcription factor (TF) CTCF is enriched at TAD boundaries and has a key role in DNA loop formation. In this period, he discovered that the transcription factor ADNP reduces CTCF occupancy at a subset of DNA binding sites and thereby locally modulates 3D genome organization.
In July 2021 Lucas Kaaij was appointed group leader at the Center for Molecular Medicine where he is continuing to unravel the complex and fascinating molecular mechanisms that shape the 3D genome.

Schematic representation of NOMe-seq (a) Schematic representation of NOMe-seq: Nuclei are isolated and accessible cytosines are methylated. DNA methylation profiles are determined by bisulfite sequencing (b) NOMe-seq discriminates between TF bound, unbound and nucleosome bound chromatin at single molecule resolution. Blue and grey circles indicate chromatin bound TFs and nucleosomes respectively.
Group members uitklapper, klik om te openen
- Lucas Kaaij – group leader
- Elke Roovers – Postdoc
Open position(s) uitklapper, klik om te openen
In the Kaaij group there is an open and collaborative environment where critical thinking and scientific creativity are highly valued.To complement our team, we currently have an open position for an enthusiastic PhD student who is interested in working on exciting problems individually or as part of a team. We are interested in applicants from a broad range of backgrounds including developmental, molecular or computational biology.
In addition, motivated master students who are interested in contributing to our efforts in understanding the 3D genome are very welcome to apply for an internship in our group. If you are interested please send an email to Lucas Kaaij.
Contact information uitklapper, klik om te openen
Key publications uitklapper, klik om te openen
Kaaij, L. J. T*#., F. Mohn*#., R. H. van der Weide, E. de Wit and M. Buhler#. (2019). The ChAHP Complex Counteracts Chromatin Looping at CTCF Sites that Emerged from SINE Expansions in Mouse. Cell
Kaaij, L. J. T*, R. H. van der Weide*, R. F. Ketting# and E. de Wit# (2018). Systemic Loss and Gain of Chromatin Architecture throughout Zebrafish Development. Cell Reports
Roovers, E. F*., L. J. T. Kaaij*, S. Redl, A. W. Bronkhorst, K. Wiebrands, A. M. de Jesus Domingues, H. Y. Huang, C. T. Han, S. Riemer, R. Dosch, W. Salvenmoser, D. Grun, F. Butter, A. van Oudenaarden and R. F. Ketting# (2018). Tdrd6a Regulates the Aggregation of Buc into Functional Subcellular Compartments that Drive Germ Cell Specification. Developmental Cell
Kaaij, L. J. T., M. Mokry*, M. Zhou*, M. Musheev, G. Geeven, A. S. Melquiond, A. M. de Jesus Domingues, W. de Laat, C. Niehrs, A. D. Smith and R. F. Ketting# (2016). Enhancers reside in a unique epigenetic environment during early zebrafish development. Genome Biology
Kaaij, L. J. T. , M. van de Wetering, F. Fang, B. Decato, A. Molaro, H. J. van de Werken, J. H. van Es, J. Schuijers, E. de Wit, W. de Laat, G. J. Hannon, H. C. Clevers, A. D. Smith and R. F. Ketting# (2013). DNA methylation dynamics during intestinal stem cell differentiation reveals enhancers driving gene expression in the villus. Genome Biology
*Indicates shared authorship
#Indicates corresponding author
