Group Rodriguez - Colman
Dr. Maria J. Rodriguez - Colman, Assistant professor

Areas of expertise uitklapper, klik om te openen
Deciphering cellular metabolism as a regulator of cell fate
We are interested in how cellular metabolism is intertwined with every process in the cell. We study the cross-talk between metabolism and cell signalling in the context of normal tissue homeostasis and in tumourigenesis. In our group we use cell lines and organoids. Organoids are derived from healthy and cancerous tissue and recapitulate in vitro, tissue architecture, cell heterogeneity and the cellular interactions that take place in vivo. We apply a number of Analytics to our research i.a.:
Live imaging of genetic encoded metabolic sensors and cell signalling reporters
Seahorse-based Bioenergetic analysis
Omics-based: Metabolomics, Proteomics and RNAseq.
Research program uitklapper, klik om te openen
Metabolism and Stem Cells
The intestinal epithelium entirely self-renews every 4-7 days. Intestinal stem cells actively divide and differentiate giving rise to all cell types in the intestine. In several tissues, metabolic changes occur along and even drive stem cell differentiation. Our research shows that in the intestine the different cell types display distinct metabolic phenotypes. As a matter of fact, we showed that mitochondrial activity is key in the maintenance of stem cell function. Currently, we apply organoid technology to investigate the cross-talk between cellular metabolism and cell signaling and how this orchestrates stem cell differentiation in normal and tumourigenic tissue.
Tumour Heterogeneity
Within a tumour, cancer cells sharing the same genetic mutations can display phenotypic differences that result in, e.g., differential response to chemotherapy. Derailed metabolism is a Hallmark of Cancer and the Warburg effect is one of the most conserved phenotypes defining tumour metabolism. Our live imaging-based single cell analysis indicates that tumour heterogeneity also occurs at the level of metabolism. We investigate the metabolism of cancer cells and how to exploit metabolic dependencies to enhance the efficacy of conventional chemotherapy.
Metabolism and Cell Cycle
The cell cycle is tightly regulated. Specific events take place in each cell cycle phase and cellular metabolism adjusts to support them. The occurrence of metabolic oscillations and their cross-talk with the cell cycle has been investigated in unicellular yeast. In our group we investigate the occurrence of metabolic fluctuations along the cell cycle and how these two events interact with each other. We study these processes both in cell lines cultures and in organotypic cultures.
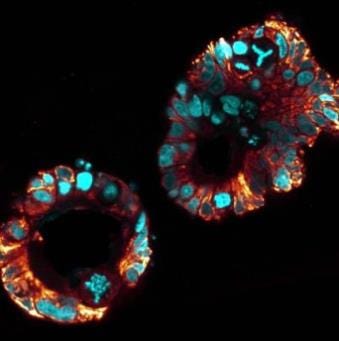
Mitochondria in tumour organoids derived from colorectal cancer patients. DNA staining shows aberrant chromosome organization (in cyan). Staining of mitochondria (in orange) indicates metabolic heterogeneity .
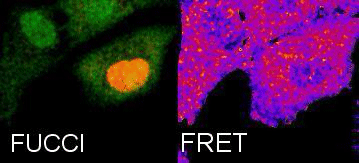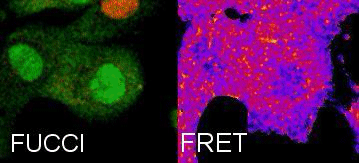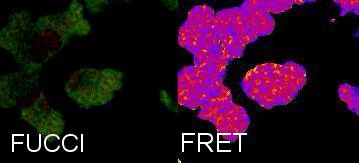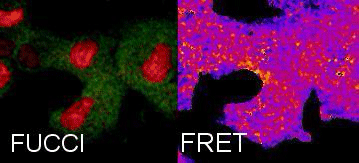
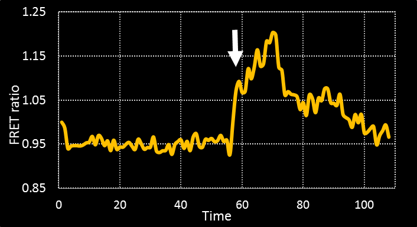
Tracking Metabolism with high temporal and spatial resolution. In our group we are interested in metabolic changes as driving forces of cell fate. We combine the FUCCI system and FRET sensors to follow metabolic oscillations during the cell cycle.
Research Group uitklapper, klik om te openen
- Marlies Ludikhuize - PhD student
- Nguyen Thi Binh Nguyen - PhD student
- Mirjam Verbeek - MSc student
Key publications uitklapper, klik om te openen
- Integrative multi-omics analysis of intestinal organoid differentiation. Lindeboom RG, van Voorthuijsen L, Oost KC, Rodríguez-Colman MJ, Luna-Velez MV, Furlan C, Baraille F, Jansen PW, Ribeiro A, Burgering BM, Snippert HJ, Vermeulen M. Mol Syst Biol. 2018 Jun 26;14(6):e8227. PMID: 29945941. IF: 10.58.
- Specific Labeling of Stem Cell Activity in Human Colorectal Organoids Using an ASCL2-Responsive Minigene. Oost KC, van Voorthuijsen L, Fumagalli A, Lindeboom RGH, Sprangers J, Omerzu M, Rodriguez-Colman MJ, Heinz MC, Verlaan-Klink I, Maurice MM, Burgering BMT, van Rheenen J, Vermeulen M, Snippert HJG. Cell Rep. 2018 Feb 6;22(6):1600-14. PMID: 2942551. IF: 8.282.
- Interplay between metabolic identities in the intestinal crypt supports stem cell function. Rodríguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Snippert HJ, Verhoeven-Duif N. Burgering BMT. Nature 2017 March; 543:424–7.
- Interplay between metabolic identities in the intestinal crypt supports stem cell function.
Rodríguez-Colman MJ, Schewe M, Meerlo M, Stigter E, Gerrits J, Pras-Raves M, Sacchetti A, Hornsveld M, Oost KC, Snippert HJ, Verhoeven-Duif N, Fodde R, Burgering BM. Nature. 2017 Mar 16;543(7645):424-427. Epub 2017 Mar 8. PMID: 28273069. IF (2017; nature.com): 41.58. - FOXOs support the metabolic requirements of normal and tumor cells by promoting IDH1 expression. Charitou P*, Rodriguez-Colman M*, Gerrits J, van Triest M, Groot Koerkamp M, Hornsveld M, Holstege F, Verhoeven-Duif NM, Burgering BM. EMBO Rep. 2015 Apr;16(4):456-66. doi: 10.15252/embr.201439096. Epub 2015 Feb 3. *Shared first authorship. PMID: 25648147. IF: 8.568
Contact Information uitklapper, klik om te openen
Visiting address:
Stratenum 3.241
Universiteitsweg 100
3584 CG Utrecht
The Netherlands
Email: M.J.RodriguezColman@umcutrecht.nl
Secretariat:
Cristina Arpesella
m.c.arpesella@umcutrecht.nl
+31 88 75 68988
