Tocilizumab effective in treating sickest COVID-19 patients
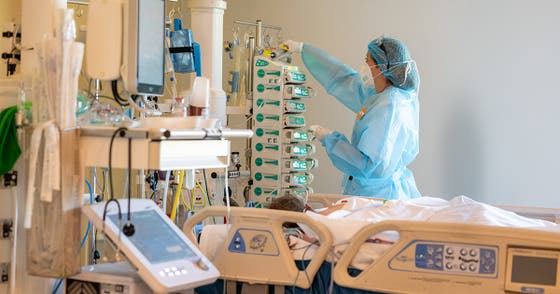
Critically ill patients with COVID-19 treated with a drug that reduces inflammation by modifying the immune system require shorter durations of organ support in the intensive care unit, an international study has found.
The early findings, which are yet to be published, come from the REMAP-CAP trial, sponsored by the UMC Utrecht in Europe and led by Imperial College London and ICNARC in the UK. They show that treatment with the immune modulator tocilizumab was 99 per cent more likely to reduce deaths and time spent in intensive care among critically ill patients with severe COVID-19, compared to patients who did not receive the treatment.
In addition, the latest analysis also revealed an antiviral drug lopinavir/ritonavir to be ineffective and provide no additional benefit to critically ill COVID-19 patients, compared to patients who received no antiviral treatment.
Due to the clinical implications for patients, the researchers have released the findings before they have been peer-reviewed but are working to analyze and publish the full results as soon as possible.
“We still need to see the full data, but this is a very exciting result”, said Doctor Lennie Derde, Consultant in Intensive Care Medicine at the University Medical Center in Utrecht, the sponsor of the study in Europe, and the Domain Specific Working Group Chair. “To have a second effective therapy for critically ill patients within months of the start of the pandemic is unprecedented. Specific targeting of the immune response is theoretically attractive, and now this indicates it works.”
The latest analysis was carried out by a Statistical Analysis Committee separate from the trial investigators and reviewed by an independent Data and Safety Monitoring Board (DSMB) on 17th November. The Analysis included data from 303 patients randomized to receive immune modulation treatments: tocilizumab, sarilumab, anakinra, interferon, or no immune modulator.
Patients receiving tocilizumab were more likely to improve (measured by a combination of organ support in the ICU and surviving the hospital admission) compared to patients who received no immune modulator. However, the trial does not yet know the relative benefits of tocilizumab compared to the other immune modulators. Further data are expected in the coming weeks and months.
The trial data yielded an estimated odds ratio of 1.87 for a better outcome with tocilizumab compared to no immune modulation, with a high degree of statistical certainty (99.75% probability that tocilizumab is superior to no immune modulation).
In addition to these findings, the latest analysis also revealed an antiviral drug called lopinavir/ritonavir to be ineffective and provide no additional benefit to critically ill COVID-19 patients, compared to those who did not receive the drug. The analysis found an estimated odds-ratio of 0.67 (worse than control) with a 99.9 per cent probability of futility (an odds ratio less than 1.20).
REMAP-CAP began investigating treatments for COVID-19 in March 2020, enrolling hospitalized patients with either moderate or severe (requiring ICU care) COVID-19 disease.
The study design randomizes patients to multiple combinations of treatments, enabling researchers to evaluate different treatments for COVID-19, including antivirals, drugs which modulate the immune response, and therapies that modulate or support other vital aspects of the body's response to the virus.
Over 2,000 patients with suspected or confirmed COVID-19 have been randomized within at least one domain, which have multiple interventions, following admission to an intensive care unit (ICU) for respiratory or cardiovascular organ support at more than 260 hospitals worldwide. The effects of interventions are assessed separately for moderate and severely ill patients.
The latest findings on tocilizumab and lopinavir/ritonavir add to REMAP-CAP findings from earlier this year, which found that hydrocortisone steroid treatment improved recovery among critically ill COVID-19 patients.
Scan de bovenstaande QR-code met uw telefoon om een video over dit onderwerp te bekijken. Of bekijk de video via:
Professor Anthony Gordon, Chair in Anaesthesia and Critical Care at Imperial College London and a Consultant in Intensive Care Medicine at Imperial College Healthcare NHS Trust, said: “These early findings show that a single course of treatment with this immune modulating drug can significantly improve the outcomes for the most critically ill COVID-19 patients in intensive care. Once we have completed the analysis of the full dataset, we hope these findings will allow critical care teams around the world to improve the outcomes of the sickest COVID-19 patients.”
The study is funded in Europe by the PREPARE consortium and RECOVER consortium Grants, and by the National Institute for Health Research (NIHR) in the UK.
This project has received funding from the European 7th Framework Programme under grant agreement No [602525].
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No [101003589].
-
REMAP-CAP
REMAP-CAP (The Randomized Embedded Multifactorial Adaptive Platform for Community Acquired Pneumonia) is an ongoing adaptive clinical trial already involving more than 2000 COVID-19 patients at more than 260 clinical sites around the world.
REMAP-CAP continues to evaluate multiple other treatments, including therapeutic anticoagulation, antiplatelet agents, apremilast, eritoran, anakinra, sarilumab, vitamin C, simvastatin, convalescent plasma, macrolides, and antibiotics.
For more information, see the UMC Utrecht website
