Stem cell therapy for brain damage in newborns
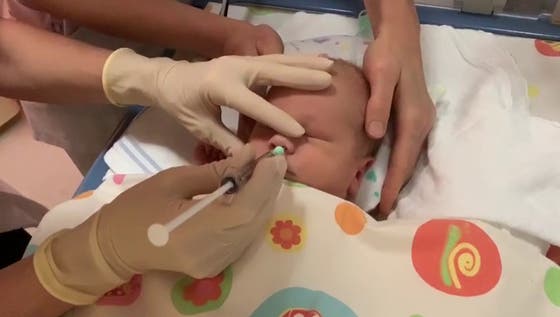
Researchers and doctors at UMC Utrecht are developing a new form of stem cell therapy for newborns with brain damage after a stroke. The treatment aims to support the natural recovery capacity of the baby brain. The clinical study recently completed shows that mesenchymal stem cells can be safely administered to these newborns via nose drops. The findings of the research groups of Cora Nijboer & Manon Benders were published in The Lancet Neurology.
The intended new treatment focuses on newborn babies with brain damage due to infarction. An infarction can be caused by a blockage of a blood vessel. It is known that these children can have problems later in life, depending on the area of the brain that is damaged. Children develop for example motor problems, learning, behaviour and concentration problems or epilepsy.
"From our lab tests, we could deduce that treatment within a few days of a blood vessel being closed is quite possible by giving stem cells." explains neuroscientist Cora Nijboer. "We have shown in animal and cellular models that damaged brain tissue is restored. We call this neuroregeneration." This treatment consists of administering so-called 'mesenchymal' stem cells. The aim is to support the affected parts of the brain, so that the brain's own stem cells are stimulated to repair the damaged tissue.
Function
Nijboer explains: "The damaged area in the brain gives off 'emergency' signals. In this way the mesenchymal stem cells are attracted to the damaged area of the brain and locally produce substances that activate the repair mechanism of the own brain stem cells." Professor of Neonatology Manon Benders adds: "That recovery is possible in newborns especially now because the brain and the networks in the brain from the third trimester of pregnancy and the first months of early life are still in full growth/development. By means of stimulation, you can also repair the remaining vital connections in damaged tissue. We call this neuroplasticity."
The stem cells are administered via nose drops. When the research began in 2010, few researchers believed that the cells would reach the damaged area of the brain in this way. After years of work by several researchers, Nijboer's team has an understanding of the route the stem cells take in a few hours. "They pass through a kind of sieve bone in the nose and are then absorbed into the blood vessels of the meninges or the cerebrospinal fluid. In this way, they migrate within a few hours to the place in the brain where the brain damage is," says Nijboer.
Safe method
Nijboer's team, together with Manon Benders' clinical team, brought the treatment to patients. Ten newborns who suffered a brain infarct participated in the study. As soon as possible after the brain infarct was detected on the MRI, they were given a single dose of stem cells via nose drops. Around the age of three months, the researchers did another MRI scan to see how much residual damage there was after the infarction. After that, the babies' development was monitored at the neonatology outpatient clinic. "In the data from this safety study, we saw signs of the efficacy of the treatment. But we need to investigate the effectiveness thoroughly, in a larger group of newborns, before we can make good, scientifically based statements about that," Benders said.
It is the first time that stem cells have been administered to newborns with brain damage via nose drops. Benders: "We kept a close eye on the ten newborns: there were no infections and no new formations on the control MRI scan, so we can conclude that this is a safe method. For us, this is an important step in the development of a new therapy, for children for whom no treatment is available so far."
"Benders and Nijboer conducted this study by successfully collaborating with the lab, the clinical department, including researchers Nienke Wagenaar and Lisanne Baak, the expertise of UMCU's cell therapy facility and the other NICUs in the Netherlands. Without the help of the other hospitals, this study would not have been possible. They moved patients to the WKZ in Utrecht specifically for the treatment, thus also demonstrating the feasibility of stem cell therapy within one week of symptoms."
Follow-up study
The doctors and researchers are aware that they have asked parents to participate in this study at a special time. "In these ten newborns, the brain damage was discovered at an early stage. Of course, this is very difficult, sad and drastic for them. But at least we can offer these parents information and intensive support," says Benders. "When a brain infarct goes undetected, the motor and cognitive consequences only become apparent after about a year. By then, the neuroplasticity of the baby brain can no longer be fully exploited." Cora Nijboer and Manon Benders are now focusing on the next steps in the research, where the emphasis will be on large-scale placebo-controlled studies to further study the therapeutic effect of the stem cells.
