Group Verhoeven/Jans
The research in our group, dedicated to discovering novel metabolic diseases and their pathophysiology, is focused more specifically on diseases related to vitamin B6 metabolism, the malate aspartate shuttle, NAD and hereditary anemias. We develop novel mass spectrometry-based technologies and bioinformatics tools to improve our understanding of these rare disorders.
Group members:
- Marjolein Bosma (research technician)
- Melissa Broeks (PhD student)
- Jolita Ciapaite (Post-doc)
- Hannah German (PhD student)
- Johan Gerrits (research technician)
- Ilja van Hoek (bio informatician)
- Judith Jans (Associate professor)
- Nils Meijer (PhD student)
- Sigrid van der Veen (PhD student)
- Nanda Verhoeven-Duif (Professor)
Vitamin B6 metabolism
The active form of vitamin B6, pyridoxal 5’-phosphate (PLP), serves as the coenzyme in at least 42 known reactions in humans, involved in amino acid and neurotransmitter metabolism, heme biosynthesis, glycogen degradation and other pathways. Several inborn errors of metabolism affecting either genes coding for enzymes involved directly in the PLP salvage pathway (PNPO, PDXK) or indirectly in the regulation of cellular PLP concentration (e.g. PLPBP, ALDH7A1, ALDH4A1) have been discovered so far.
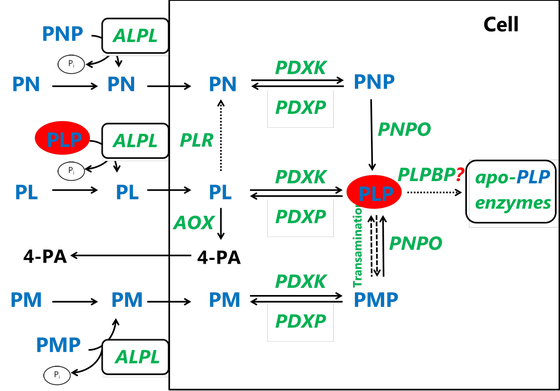
Genetic defects affecting vitamin B6 metabolism usually manifest in encephalopathy, seizures, neurodevelopmental delay, or neuropathy. Early diagnosis can be lifesaving and contributes to better treatment outcomes. In our group, we are investigating these disorders in various model systems, including patient body fluids (plasma, CSF), patient derived skin fibroblasts, specific gene knock-out cell and zebrafish lines generated using CRISPR/Cas9 gene editing. Furthermore, in collaboration with the group of prof. dr. Natal van Riel (TU/e) we are working on implementing PLP cofactor in human genome-scale metabolic models to investigate inborn errors in vitamin B6 metabolism in silico. Our aim is to understand and gain new insights in molecular mechanisms underlying pathophysiology of these diseases, to test new treatment strategies and to discover new diagnostic biomarkers.
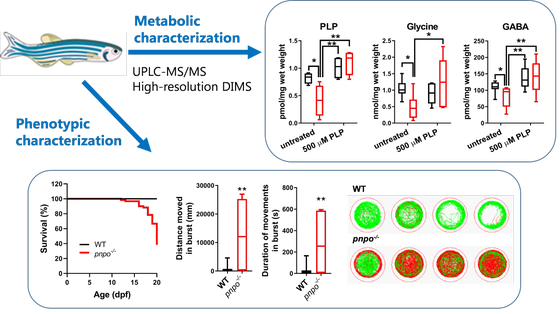
The malate aspartate shuttle
The malate aspartate shuttle (MAS) is a metabolic pathway that transports reducing equivalents in the mitochondrial matrix, while simultaneously maintaining cytosolic NAD+/NADH redox balance. We focus on elucidating the underlying pathophysiological mechanism in deficiencies in all 6 components of the MAS and subsequently exploring the effects of novel therapeutics using metabolomics and stable isotope tracer analysis.
Ultimately, aiming to find diagnostic markers and opportunities for treatment to improve patientcare.
NAD biosynthesis and fluxomics
Recently, defects in NAD biosynthesis have been discovered. We investigate these defects in vitro using mass spectrometry-based methods.
Hereto, we developed a novel workflow aimed at characterizing metabolic fluxes in untargeted metabolomics data derived from direct infusion high resolution mass spectrometry (DI-HRMS).
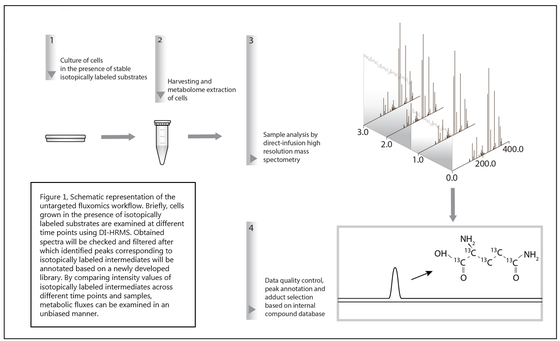
Hereditary anemias
One of the focus points within hereditary anemias is sickle cell disease (SCD), a hemoglobinopathy. We focus on identifying new biomarkers for the prediction of the disease severity and phenotype in SCD. Simultaneously, aiming to improve our understanding of the pathophysiology in SCD. Previously, we identified untargeted metabolomic fingerprints in dried blood spots of hereditary anemia patients to evaluate the potential of this approach for diagnostic and pathophysiological insights. This potential was demonstrated in combination with machine learning analysis for pyruvate kinase deficiency (PKD), hereditary spherocytosis (HS), and the ribosomal biogenesis defect Diamond Blackfan Anemia (DBA).
