U-TRIAL and innovations in trial methodology
At U-TRIAL, we strengthen the UMC Utrecht clinical trial ecosystem. We do so, for instance by helping further innovations in trial methodology.
Randomized trials are the gold standard for making statements about the comparative efficacy and safety of new interventions in health care. In recent years, both investigator-initiated and industry-sponsored studies have become more and more expensive, and it generally takes a long time before final results become available. Also, the representativeness of subjects included in clinical trials is often a point of discussion, as their often strict inclusion criteria make that the population included does not reflect the intended use population. Finally, the standard randomized clinical trials are less suitable for studying the increasing number of personalized therapies and therapies targeting rare disease indications. To keep clinical trials feasible, make them more inclusive in the future and more suitable for evaluating modern types of therapies, innovations in trial design and methodology are clearly needed. Uptake and implementation of these innovations in practice needs to be stimulated and supported. This is an important focus of U-TRIAL, which we share with various national and international initiatives, such as Accelerating Clinical Trials in the EU (ACT-EU).
Innovative trial designs and methodology
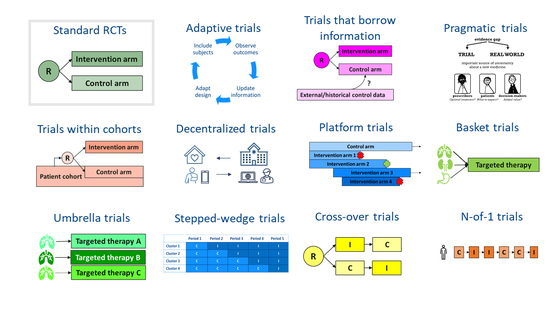
Relevant examples of recent innovations in clinical trial methodology include
- adaptive trials
- trials that borrow information from previous trials or real world data
- pragmatic trials
Adaptive trials aim at increasing the efficiency of trials by using outcomes observed during the trial to further optimize the trials. Data-driven decisions can be incorporated, such as early stopping for futility or overwhelming efficacy, further restricting the trial to a subpopulation that is responsive to treatment or re-estimating the required sample size. Adaptive randomization may be used to increase the proportion of trial participants randomized to the best performing new interventions.
Dynamic borrowing methods aim to make efficient use of available data from patients on control treatments in previous trials or available real-world data, and use their data to enrich the control arm in a new trial. These techniques weigh the historical data based on their similarity to the controls in the trial. These methods may potentially reduce the number of patients that need to be randomized to control arms, which is of particular relevance for trials in rare indications.
Pragmatic trials aim to recruit the general population of patients who would receive the intervention if it became usual care, making trials more inclusive and results more representative for targeted patient populations. These are just a few examples. See more trial designs in the graphic below.
Involvement of trial methodologists is more important than ever
U-TRIAL actively supports knowledge exchange and uptake of innovations in clinical trial methodology, through dissemination of innovative trial methodologies at internal meetings and connecting investigators and trial methodologists. The trial methodologists at UMC Utrecht have experience with a wide range of innovative trial designs including those represented in the graphic. The increased complexity of the newer trial designs and associated analyses makes early involvement of trial methodologists a prerequisite for trial success.
Connect with Us
Interested in learning more about how U-TRIAL can support your research? Reach out to our team at u-trial@umcutrecht.nl.
